Key requirements for a CoA to be used as a reference standard is:
(1) Data that confirms its molecular structure identity (this is an absolute requirement); and
(2) Its potency value (this is different than its purity value!)
Additional analysis are required to probe for trace toxic and biologics if a compound will be administered to human or animals.
Kindly note that all Chemtos products are Certified Analytical Reference Standards that may contain minor or trace amounts of unidentified impurities (hence our products are not GMP certified).
A presentation that discusses various aspects of CoA can be found in the link below:
Link to: Powerpoint presentation on CoA requirements for reference standards
Are you using poorly characterized (and potentially unacceptable) reference standards? Consider whether a CoA with just TLC or limited characterization is adequate for your application.
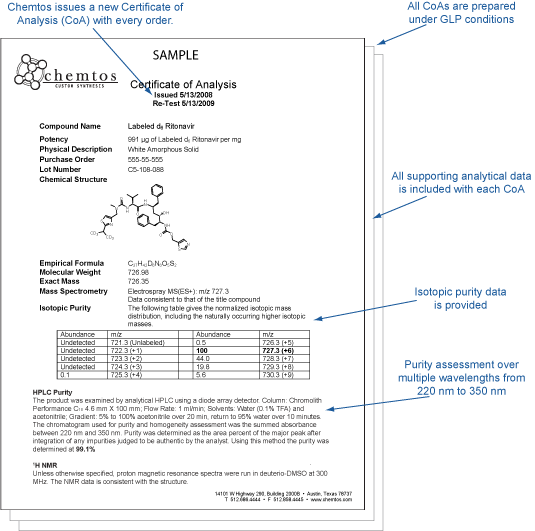
With our comprehensive certification, you will never need to doubt the authenticity or purity of our or your reference standards. Every compound provided by Chemtos comes fully certified whose identity is confirmed using LC/MS and Proton NMR, purity confirmed using UV HPLC, and accurate potency determined using Quantitative proton NMR analysis. Copies of all supporting analytical data is included in the CoA, so there is no doubt. All analytical work is done following GLP protocols and SOPs. See a sample CoA.
We also offer our analytical expertise and services to re-certify and accurately determine potency using qNMR of your reference standard compounds, formulations, or solutions in volatile solvents such as methanol or acetonitrile.