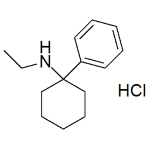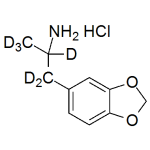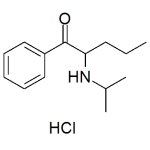Chemtos
Service, Quality, Speed
Chemtos
Service, Quality, Speed
Ref Stds
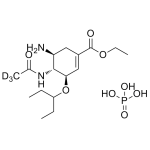
Oseltamivir Phosphate Labeled d3
High purity Oseltamivir Phosphate Labeled d3 includes a comprehensive Certificate of Analysis and all supporting analytical data
Hydromorphone 1mg/ml
High purity Hydromorphone solution includes a comprehensive Certificate of Analysis and all supporting analytical data
Asenapine-N-glucuronide
High purity Asenapine-N-glucuronide includes a comprehensive Certificate of Analysis and all supporting analytical data
Amitriptyline HCl 1mg/ml
High purity Amitriptyline HCl solution includes a comprehensive Certificate of Analysis and all supporting analytical data
JWH-019
High purity JWH-019 includes a comprehensive Certificate of Analysis and all supporting analytical data
4-Hydroxy-Alprazolam
High purity 4-Hydroxy-Alprazolam includes a comprehensive Certificate of Analysis and all supporting analytical data
Norsertraline labeled d7 Hydrochloride
High purity Norsertraline-d7 Hydrochloride / N-Desmethylsertraline-d7 Hydrochloride includes a comprehensive Certificate of Analysis and all supporting analytical data
JWH-203
High purity JWH-203 (1-Pentyl-3-(2-chlorophenylacetyl) indole) includes a comprehensive Certificate of Analysis and all supporting analytical data
Eticyclidine (PCE) HCl 1mg/ml
High purity Eticyclidine HCl (PCE, N-Ethyl-1-phenylcyclohexylamine, CI-400, PCPEt, Cyclohexamine, DEA C-I #7455) solution includes a comprehensive Certificate of Analysis and all supporting analytical data
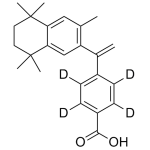
Bexarotene Labeled d4
High purity Bexarotene Labeled d4 includes a comprehensive Certificate of Analysis and all supporting analytical data
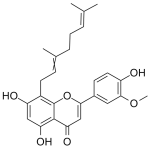
Cannflavin C
High purity Cannflavin C includes a comprehensive Certificate of Analysis and all supporting analytical data
MDA-d6 HCl 0.1mg/ml
High purity MDA labeled-d6 HCl solution includes a comprehensive Certificate of Analysis and all supporting analytical data
NiPP HCl 1mg/ml
High purity NiPP HCl (2-Isopropylamino)-1-phenylpentan-1-one, 2-IPP, α-Isopropylaminopentiophenone, IPV, N-iPr-norpentedrone) HCl solution includes a comprehensive Certificate of Analysis and all supporting analytical data
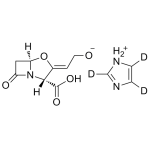
Clavulanic acid Imidazole-d3 salt
High purity Clavulanic acid Imidazole-d3 salt includes a comprehensive Certificate of Analysis and all supporting analytical data
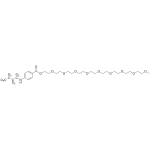
Benzonatate Labeled d9
High purity Benzonatate Labeled d9 includes a comprehensive Certificate of Analysis and all supporting analytical data